MODEYSO demonstrated clinical benefit1
Response was observed across a range of measures in patients with recurrent H3 K27M–mutant diffuse midline glioma1
INTEGRATED ANALYSIS DESIGN
The efficacy and safety of MODEYSO were evaluated in an integrated analysis1
The MODEYSO integrated analysis was designed in alignment with the FDA, generating a population of patients with H3 K27M–mutant diffuse midline glioma (DMG) sufficient to determine safety and efficacy.1,2

To isolate single-agent activity, a pooled analysis of 50 adult and pediatric patients with H3 K27M–mutant DMG was conducted using dual-reader BICR and RANO 2.0 criteria. These patients were drawn from 5 clinical studies evaluating MODEYSO.†
Major efficacy outcome:
Overall response rate (ORR)
Additional efficacy outcomes:
Duration of response (DoR),
time to response (TTR)

The safety of MODEYSO was evaluated in 376 adult and pediatric patients with glioma across 4 open-label clinical studies. Patients in the safety cohort received MODEYSO once-weekly at a dose of 625 mg or scaled by body weight for patients weighing <52.5 kg.
Safety was evaluated across 154 pediatric patients and 222 adult patients of different races and ethnicities.
See additional characteristics of the safety cohort
BICR, blinded independent central review; FDA, US Food and Drug Administration; H3, histone 3; RANO, Response Assessment in Neuro-Oncology.
*ONC016 was a compassionate use program, and patients enrolled in this study were excluded from the safety cohort due to differences in data collection protocols.2
†With the exception of one patient who received MODEYSO once every 3 weeks, all patients received once-weekly MODEYSO at a dose of 625 mg (or scaled by body weight for pediatric patients weighing <52.5 kg).1,2
- Patients aged ≥2 years
- Diffuse midline glioma with a confirmed H3 K27M mutation
- Progressive and measurable disease on contrast-enhanced MRI by RANO-HGG criteria
- Adequate washout from prior anticancer therapies. See specified washout periods by therapy
- KPS/LPS score ≥60.‡ See scale descriptions for performance score
- Stable to decreasing corticosteroid dose for ≥3 days prior to baseline scan
- Received oral MODEYSO as monotherapy. See body weight–adjusted dosing
- DIPG
- Leptomeningeal spread
- Primary spinal tumors
- Cerebrospinal fluid dissemination
- Atypical and nonastrocytic histologies
DIPG, diffuse intrinsic pontine glioma; HGG, high-grade glioma; KPS/LPS, Karnosfsky Performance Status or Lansky Performance Status, depending on age; MRI, magnetic resonance imaging.
‡Performance status measured using KPS (patients aged ≥16 years) and LPS (patients aged ≥1 year and <16 years). All patients (N=50) had a score of ≥60 at baseline.1,3
POPULATION DEMOGRAPHICS
- Median age: 31 years
- Range: 9 to 70 years
- 6% of patients under 17 years
- 46% female, 54% male
- Race
- 80% White
- 10% other racial groups
- 6% Black/African American
- 2% Asian
- 2% unknown or not reported
- Ethnicity
- 8% Hispanic/Latino
- 72% of patients had a KPS/LPS score of 80 to 100‡
- 72% of patients were treated at first recurrence
- 28% had 2 or more recurrences
- 52% had primary tumor located in the thalamus
- 48% had primary tumor located in non-thalamic midline region
- 88% received prior temozolomide
- 62% were receiving corticosteroids at baseline
- Median time since prior radiation: 7.4 months (range: 3-102.1 months)
CLINICAL EFFICACY DATA
MODEYSO demonstrated clinical benefit in patients with recurrent H3 K27M–mutant diffuse midline glioma1
Major efficacy outcome (ORR) measured with RANO 2.0 by BICR assessment (N=50)1
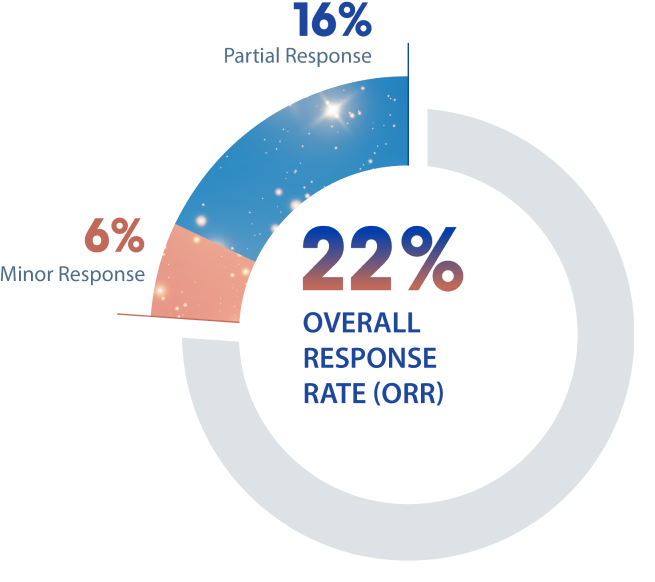
22% of patients (n=11; 95% CI: 12-36) achieved an overall response by RANO 2.0 criteria§
- 8 patients experienced a partial response
- 3 patients experienced a minor response
Using BICR-assessed RANO 2.0 criteria, there was 1 additional responder based on the integrated response assessment, which takes into account corticosteroid use and performance status.1
RANO 2.0 response qualifications
Partial response included a ≥50% decrease in the product of perpendicular diameters or ≥65% decrease in total volume of either the contrast-enhancing target lesion(s) or the T2 or FLAIR target lesion(s), sustained for at least 4 weeks and with no increase in corticosteroid dose.4
Minor response included a 25% to 50% decrease in the sum of the products of perpendicular diameters or between 40% and 65% decrease in the total volume of non-enhancing target lesion(s) on T2 or FLAIR MRI compared with baseline, sustained for at least 4 weeks and with no increase in corticosteroid dose.4
CI, confidence interval; FLAIR, fluid-attenuated inversion recovery.
§CI based on Clopper-Pearson method.1
Patients who responded to MODEYSO achieved sustained results1
Additional efficacy outcomes: time to response and duration of response among patients who achieved an overall response to MODEYSO1

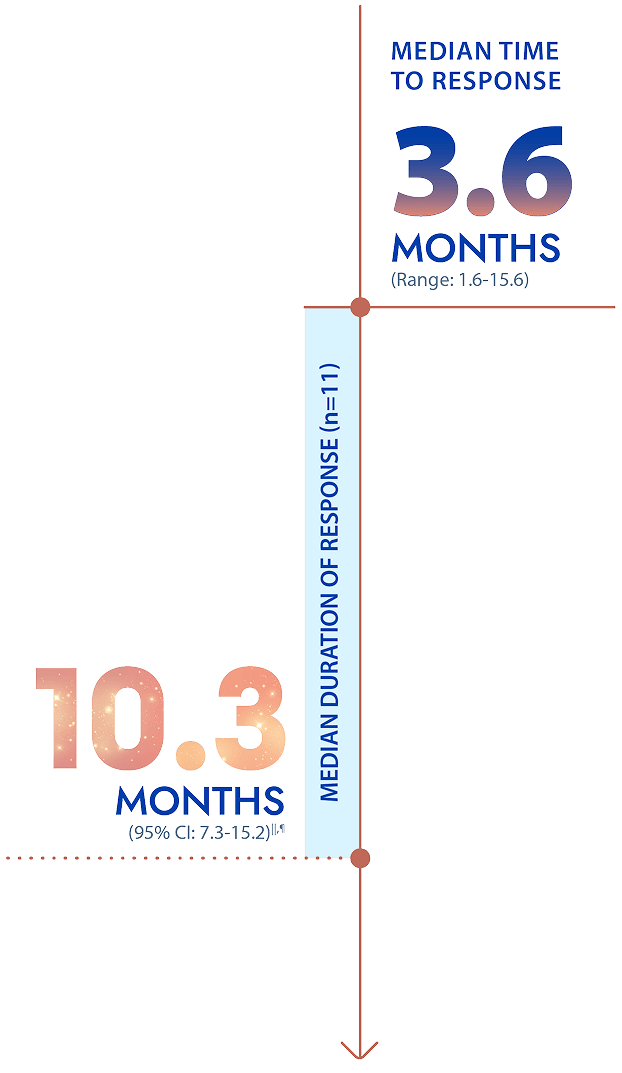
||Based on Kaplan-Meier estimate.1
¶Duration of response is defined as time from first response per RANO criteria to disease progression or death.1,5
CONNECT
Talk with a representative to learn how MODEYSO could expand possibilities for your patients1
References: 1. MODEYSO™. Package insert. Jazz Pharmaceuticals, Inc; 2026. 2. Arrillaga-Romany I, Gardner SL, Odia Y, et al. ONC201 (dordaviprone) in recurrent H3 K27M–mutant diffuse midline glioma. J Clin Oncol. 2024;42(13):1542-1552. doi:10.1200/JCO.23.01134 3. CIBMTR. Forms Instruction Manual. CIBMTR; updated January 27, 2025. https://cibmtr.org/CIBMTR/Data-Operations/Manuals-Guides 4. Wen PY, van den Bent M, Youssef G, et al. RANO 2.0: update to the Response Assessment in Neuro-Oncology criteria for high- and low-grade gliomas in adults. J Clin Oncol. 2023;41(33):5187-5199. doi:10.1200/JCO.23.01059 5. Arrillaga-Romany I, Gardner SL, Odia Y, et al. ONC201 (dordaviprone) in recurrent H3 K27M–mutant diffuse midline glioma. J Clin Oncol. 2024;42(suppl protocol):1-317. doi:10.1200/JCO.23.01134